Research projects
RESEARCH SUBJECTS OF THE SCHMIDT GROUP
1. N-Heterocyclic carbenes from mesomeric betaines
Mesomeric betaines are conjugated molecules which can exclusively be represented by dipolar canonical formulae. Five distinct types of conjugation can be distiguished which govern the chemical and physical properties of this class of compounds. Their conversions into N-heterocyclic carbenes yield normal, abnormal, and remote NHCs depending on the type of conjugation of the betaine precursors. Reviews summarize results (1. A. Schmidt, S. Wiechmann, T. Freese, ARKIVOC 2013, i, 424 - 469. 2. A. Schmidt, S. Wiechmann, C. F. Otto, Adv. Heterocycl. Chem. 2016, 119, 143 - 172.). Mayor routes from mesomeric betaines to N-heterocyclic carbenes include decarboxylations from pseudo-cross-conjugated hetarenium-carboxylates, tautomerizations, and deprotonations. The latter result in the formation of anionic N-heterocyclic carbenes with unusual properties which essentially originate from considerable atomic orbital coefficients of the highest occupied molecular orbital (HOMO), which is a pi-orbital, at the carbene carbon atom. The HOMO energies of these systems indicate very electron-rich NHCs.They form a broad varitey of complexes and can be applied as catalysts in Suzuki-Miyaura reactions in acid.
a) Anionic N-heterocyclic carbenes from sydnones and derivatives
S. Mummel, F. Lederle, E. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt, Angew. Chem. Int. Ed. 2021, 60, 18882 - 18887; Angew. Chem.2021, 133, 19032 - 19037.
T. Freese, A.-L. Lücke, J. C. Namyslo, M. Nieger, A. Schmidt, Eur. J. Org. Chem. 2018, 1646 - 1654.
A.-L. Lücke, S. Wiechmann, T. Freese, M. Nieger, T. Földes, I. Pápai, M. Gjikaj, A. Adam, A. Schmidt, Tetrahedron2018, 74, 2092 - 2099.
b) Complexes of anionic N-heterocyclic carbenes derived from mesomeric betaines (Au, Pd, Hg, B, Se, Rh, Ni...)
K. Hillrichs, J. C. Namyslo, F. Lederle, E. G. Hübner, A. Schmidt, Synthesis2022, 3351 - 3366.
T. Freese, A.-L. Lücke, C. A. S. Schmidt, M. Polamo, M. Nieger, J. C. Namyslo, A. Schmidt, Tetrahedron 2017, 73, 4472 - 4480.
M. Liu, J. C. Namyslo, M. Nieger, M. Polamo, A. Schmidt, Beilstein J. Org. Chem. 2016, 12, 2673 - 2681.
M. Liu, M. Nieger, E. Hübner, A. Schmidt, Chem. Eur. J. 2016, 5416 - 5424.
c) Catalysis: Suzuki-Miyaura reactions under classical and acidic conditions
A.-L. Lücke, L. Pruschinski, T. Freese, A. Schmidt, ARKIVOC2020, VII, 94 - 104.
A.-L. Lücke, S. Wiechmann, T. Freese, A. Schmidt, Synlett2017, 28, 1990 - 1993.
S. Wiechmann, T. Freese, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt, Chem. Commun. 2014, 50, 11822 - 11824.
A. Rahimi, J. C. Namyslo, M. Drafz, J. Halm, E. Hübner, M. Nieger, N. Rautzenberg, A. Schmidt, J. Org. Chem. 2011, 76, 7316 - 7325.
A. Schmidt, A. Rahimi, Chem. Comm. 2010, 46, 2995 - 2997.
A. Rahimi, A. Schmidt, Synlett2010, 1327 - 1330.
A. Rahimi, A. Schmidt, Synthesis2010, 2621 - 2625.
d) N-Heterocyclic carbenes as tautomers of mesomeric betaines
J. Zhang, E. G. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt, Org. Biomol. Chem. 2018, 16, 6801 - 6808.
e) Triple bonds in mesomeric betaines. A world of surprises.
S. Batsyts, R. Vedmid, J. C. Namyslo, M. Nieger, A. Schmidt, Eur. J. Org. Chem. 2019, 1301 - 1310.
A. Schmidt, S. Batsyts, A. Smeyanov, T. Freese, E. G. Hübner, M. Nieger, J. Org. Chem. 2016, 81, 4202 - 4209.
2. New photoresponsive and redox-active resins and polymers inspired by Nature
Based on the alkaloid punicin from Punica granatum, which is a mesomeric betaine, we prepared a variety of photoresponsive and redox-active monomers and polymers as well as resins. These materials can serve as molecular switches, as visible light changes the chemical and physical properties reversibly. Application in recycling is currently investigated.
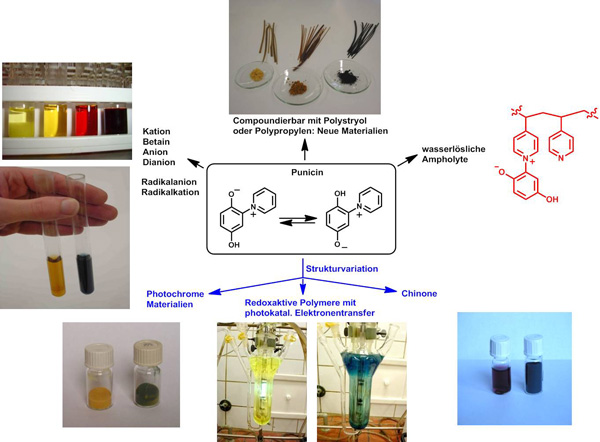
C. F. Otto, M. Liu, C. Herzberger, J. C. Namyslo, M. Nieger, E. G. Hübner, F. Lederle, T. Freese, A. Schmidt, Tetrahedron2020, 76, 131627.
A. Schmidt, M. Albrecht, T. Mordhorst, C. F. Otto, H. Fleischhauer, M. Topp, Bull. Mater. Res. Engin. 2015, 318 - 326.
M. Albrecht, M. Yulikov, T. Kohn, G. Jeschke, J. Adams, A. Schmidt, J. Mater. Chem. 2010, 20, 3025 - 3034.
M.Albrecht, O. Schneider, A. Schmidt, Org. Biomol. Chem. 2009, 7, 1445 - 1453.
A. Schmidt, M. Albrecht, T. Mordhorst, M. Topp, G. Jeschke, J. Mater. Chem.2007, 17, 2793 - 2800.
A. Schmidt, M. Topp, T. Mordhorst, O. Schneider, Tetrahedron2007, 63, 1842 - 1848.
3. Fluorescent borane adducts of N-heterocyclic carbenes

J. Zhang, E. G. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt, Org. Biomol. Chem. 2018, 16, 6801 - 6808.
J. Zhang, M. Franz, E. Hübner, A. Schmidt, Tetrahedron 2016, 72, 525 - 531.
M. Liu, M. Nieger, A. Schmidt, Chem. Commun. 2015, 51, 477 - 479.
N. Pidlypnyi, J. C. Namyslo, M. H. H. Drafz, M. Nieger, A. Schmidt, J. Org. Chem. 2013, 78, 1070 - 1078.
N. Pidlypnyi, F. Uhrner, M. Nieger, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, A. Schmidt, Eur. J. Org. Chem. 2013, 7739 - 7748.
4. Chemistry of aromatics with polycations
S. Batsyts, J. C. Namyslo, E. Hübner, A. Schmidt, Eur. J. Org. Chem. 2019, 6168 - 6176.
S. Batsyts, F. Lederle, E. G. Hübner, J. Adams, J. C. Namyslo, A. Schmidt, Heterocycles2019, 98, 1445 - 1454.
J. Hiller, M. Liu, A. Schmidt, Heterocycles2017, 94, 821 - 858.
A. Schmidt, T. Mordhorst, Synthesis 2005, 781 - 786.
5. Organic chemistry on surfaces - cooperation with Prof. Maus-Friedrichs and Dr. Höfft
M. Marschewski, H. Tas, C. Otto, W. Maus-Friedrichs, A. Schmidt, O. Höfft, J. Electron Spectr. Rel. Phen. 2018, 229, 26 - 32.
M. Marschewski, C. Otto, L. Wegewitz, O. Höfft, A. Schmidt, W. Maus-Friedrichs, Appl. Surface Sci. 2015, 339, 9 - 14.
6. Building blocks of metal organic frameworks (MOFs)
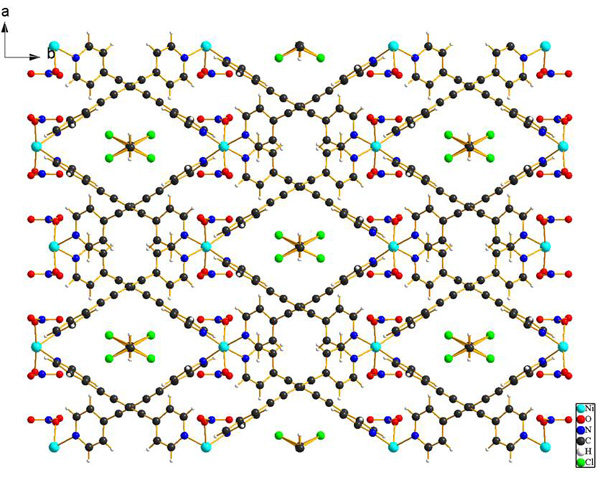
A. Smeyanov. M. Nieger, R. Gustus, W. Maus-Friedrichs, A. Schmidt, Z. Naturforsch. 2015, 70b, 897 - 902.
M. Albrecht, M. Nieger, A. Schmidt, Z. Naturforsch. 2012, 67b, 103 - 106.
M. Albrecht, M. Nieger, A. Schmidt, Z. Naturforsch. 2011, 66b, 209 - 212.
7. New Bis(thienyl)ethenes for technical applications
S. Nagorny, T. Weingartz, J. C. Namyslo, J. Adams, A. Schmidt, Eur. J. Org. Chem. 2022, submitted.
S. Nagorny, F. Lederle, V. Udachin, T. Weingartz, E. G. Hübner, S. Dahle, W. Maus-Friedrichs, J. Adams, A.
Schmidt, Eur. J. Org. Chem. 2021, 3178 - 3189.
