Research
We are interested to develop and apply new chiral organic salts. The work is focusing mainly on salts containing imidazolinium and amidinium cations. These species are less represented in the literature compared to their unsaturated imidazolium analogues, despite the fact that they have high potential in many applications. We explore the potential of these salts as chiral ionic liquids and carbene precursors.
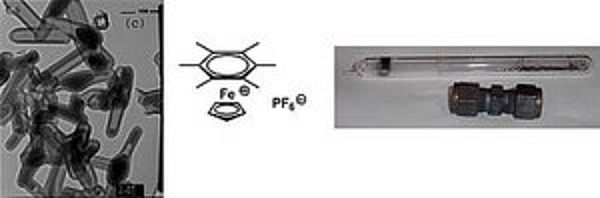
We are involved in the synthesis of new carbon-nanomaterials, which could have for example a high potential for the application in heterogeneous catalysis or drug delivery systems. Therefore, organic salts based on CpFe(arene) cations are used to develop new routes to new carbon nanostructures on a large scale. In order to prepare the novel nanostructures, the salts are pyrolysed. Different metal complexes can give various nanostructures of carbon, like carbon nanotubes, fibers and other forms. An example for the synthesis of carbon nanoparticles via pyrolysis of an organometallic compound is depicted on the left side.
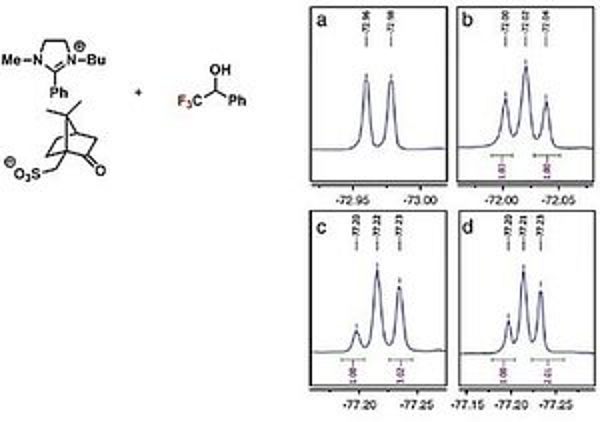
Ionic liquids have by definition a melting point below 100 °C. These liquids have a high potential as “green solvents”, e.g. due to their negligible vapour pressure and efficient recovery. Chiral ionic liquids have an additional potential as chiral solvents, shift reagents and catalysts. We develop new salts in order to apply them in chiral recognition as chiral NMR solvent and in catalysis for the syntheses of intermediates of biological active compounds. An example of a chiral ionic liquid as chiral NMR solvent is depicted on the left side.
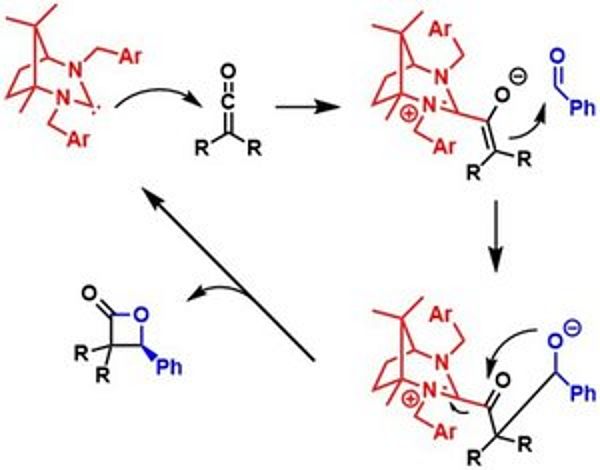
Carbenes have a neutral and bivalent carbon atom with a sextet of electrons. They can be for example prepared in situ from amidinium salts via deprotonation. We develop new chiral amidinium salts from inexpensive compounds from the “Chiral Pool” to generate very nucleophilic carbenes as organocatalysts for the synthesis of biological active compounds like β-lactones. In addition, we explore these carbenes also as ligands for metal catalyzed asymmetric reaction. Since the amidinium salt derived carbenes are very strong s-donors, stable complexes can be obtained, which is beneficial for high turnover numbers. An example of a chiral carbene as organocatalyst in a Wynberg reaction is depicted on the left side.