RESEARCH SUBJECTS OF THE SCHMIDT GROUP
1. N-Heterocyclic carbenes from mesomeric betaines
What are mesomeric betaines? These are conjugated molecules that can only be represented by dipolar canonical formulas. This is what makes them so fascinating to this day. Mesomeric betaines include mesoionic compounds such as sydnones and münchnones. At least five different types of conjugation can be distinguished, which determine the chemical and physical properties of this class of compounds. Depending on the type of conjugation of the betaine precursors, their conversion to N-heterocyclic carbenes leads to normal, abnormal, and distant NHCs. Important pathways from mesomeric betaines to N-heterocyclic carbenes include decarboxylations from pseudo-cross-conjugated hetarenium carboxylates, tautomerizations, and deprotonations. The latter lead to the formation of anionic N-heterocyclic carbenes with unusual properties, which are essentially due to considerable atomic orbital coefficients of the highest occupied molecular orbital (HOMO), a pi orbital, at the carbene carbon atom. The HOMO energies of these systems indicate very electron-rich NHCs. They form a variety of complexes and can be used as catalysts in Suzuki-Miyaura reactions in acid.
2. New photoresponsive and redox-active molecules, resins and polymers inspired by Nature for recycling of critical raw materials
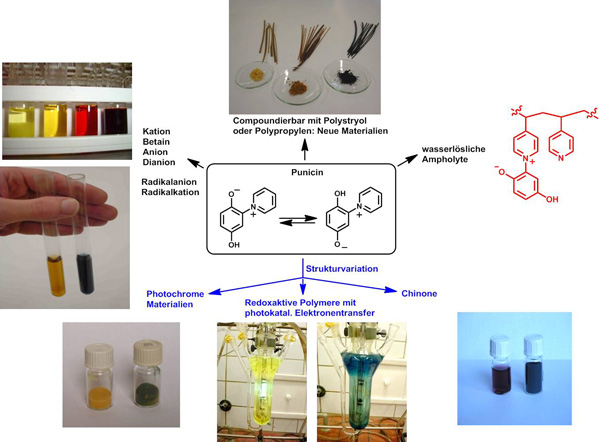
Based on the alkaloid punicin from Punica granatum, which is a mesomeric betaine, we prepared a variety of photoresponsive and redox-active monomers and polymers as well as resins. These materials can serve as molecular switches, as visible light changes the chemical and physical properties reversibly. Application in recycling is currently being investigated.
C. F. Otto, M. Liu, C. Herzberger, J. C. Namyslo, M. Nieger, E. G. Hübner, F. Lederle, T. Freese, A. Schmidt, Tetrahedron2020, 76, 131627.
A. Schmidt, M. Albrecht, T. Mordhorst, C. F. Otto, H. Fleischhauer, M. Topp, Bull. Mater. Res. Engin. 2015, 318 -326.
M. Albrecht, M. Yulikov, T. Kohn, G. Jeschke, J. Adams, A. Schmidt, J. Mater. Chem. 2010, 20, 3025 - 3034.
M. Albrecht, O. Schneider, A. Schmidt, Org. Biomol. Chem. 2009, 7, 1445 - 1453.
A. Schmidt, M. Albrecht, T. Mordhorst, M. Topp, G. Jeschke, J. Mater. Chem.2007, 17, 2793 - 2800.
A. Schmidt, M. Topp, T. Mordhorst, O. Schneider, Tetrahedron2007, 63, 1842 - 1848.
3. fluorescent borane adducts of N-heterocyclic carbenes
J. Zhang, E. G. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt, Org. Biomol. Chem. 2018, 16, 6801 - 6808.
J. Zhang, M. Franz, E. Hübner, A. Schmidt, Tetrahedron 2016, 72, 525 - 531.
M. Liu, M. Nieger, A. Schmidt, Chem. Commun. 2015, 51, 477 - 479.
N. Pidlypnyi, J. C. Namyslo, M. H. H. Drafz, M. Nieger, A. Schmidt,J. Org. Chem. 2013,78, 1070 - 1078.
N. Pidlypnyi, F. Uhrner, M. Nieger, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, A. Schmidt, Eur. J. Org. Chem. 2013, 7739 - 7748.
4 Chemistry of aromatics with polycations
S. Batsyts, J. C. Namyslo, E. Hübner, A. Schmidt, Eur. J. Org. Chem. 2019, 6168 - 6176.
S. Batsyts, F. Lederle, E. G. Hübner, J. Adams, J. C. Namyslo, A. Schmidt, Heterocycles2019, 98, 1445 - 1454.
J. Hiller, M. Liu, A. Schmidt, Heterocycles2017, 94, 821 - 858.
A. Schmidt, T. Mordhorst, Synthesis 2005, 781 - 786.
5.Organic chemistry on surfaces - cooperation with Prof. Maus-Friedrichs and Dr. Höfft
M. Marschewski, H. Tas, C. Otto, W. Maus-Friedrichs, A. Schmidt, O. Höfft, J. Electron Spectr. Rel. Phen. 2018, 229, 26 - 32.
M. Marschewski, C. Otto, L. Wegewitz, O. Höfft, A. Schmidt, W. Maus-Friedrichs, Appl. Surface Sci. 2015, 339, 9 - 14.
6. building blocks of metal organic frameworks (MOFs)
A. Smeyanov. M. Nieger, R. Gustus, W. Maus-Friedrichs, A. Schmidt, Z. Naturforsch. 2015, 70b, 897 - 902.
M. Albrecht, M. Nieger, A. Schmidt, Z. Naturforsch. 2012, 67b, 103 - 106.
M. Albrecht, M. Nieger, A. Schmidt, Z. Naturforsch. 2011, 66b, 209 - 212.
7 New bis(thienyl)ethenes for technical applications
S. Nagorny, T. Weingartz, J. C. Namyslo, J. Adams, A. Schmidt, Eur. J. Org. Chem. 2022, submitted.
S. Nagorny, F. Lederle, V. Udachin, T. Weingartz, E. G. Hübner, S. Dahle, W. Maus-Friedrichs, J. Adams, A.
Schmidt, Eur. J. Org. Chem. 2021, 3178 - 3189.