Research projects
RESEARCH SUBJECTS OF THE SCHMIDT GROUP
1. N-Heterocyclic carbenes from mesomeric betaines
What are mesomeric betaines? They are conjugated molecules that can only be represented by dipolar canonical formulas. This is what makes them so fascinating to this day. The class of mesomeric betaines includes mesoionic compounds such as sydnones and münchnones. At least five different types of conjugation can be distinguished, which determine the chemical and physical properties of this class of compounds. Their conversion to N-heterocyclic carbenes leads to normal, abnormal, and remote NHCs, depending on the type of conjugation of the betaine precursors. Important pathways from mesomeric betaines to N-heterocyclic carbenes include decarboxylations from pseudo-cross-conjugated hetarenium carboxylates, tautomerizations, and deprotonations. The latter lead to the formation of anionic N-heterocyclic carbenes with unusual properties, which are essentially due to considerable atomic orbital coefficients of the highest occupied molecular orbital (HOMO), a pi orbital, at the carbene carbon atom. The HOMO energies of these systems indicate very electron-rich NHCs. They form a variety of complexes and can be used as catalysts in Suzuki-Miyaura reactions in acid (!).
2. N-Heterocyclic olefins of pyrazole and indazole
N-Heterocyclic olefins (NHOs) are related to N-heterocyclic carbenes (NHCs). They can therefore be regarded as methylene adducts of the corresponding carbenes. N-heterocyclic olefins are at the beginning of a promising career – at least, the results available so far are very encouraging. We are working on N-heterocyclic carbenes of pyrazole and indazole, which differ in many ways from their previously known relatives. This also ties in with a scientific tradition in our research group: N-heterocyclic carbenes of pyrazole and indazole have been studied intensively in our group.
3. New photoresponsive and redox-active molecules, resins and polymers inspired by Nature for recycling (SPP 2315 of the DFG)
Based on the alkaloid punicine from Punica granatum, which is a mesomeric betaine, we prepared a variety of photoresponsive and redox-active monomers and polymers as well as resins. These materials can serve as molecular switches, as visible light changes the chemical and physical properties reversibly. Application in recycling is currently investigated.
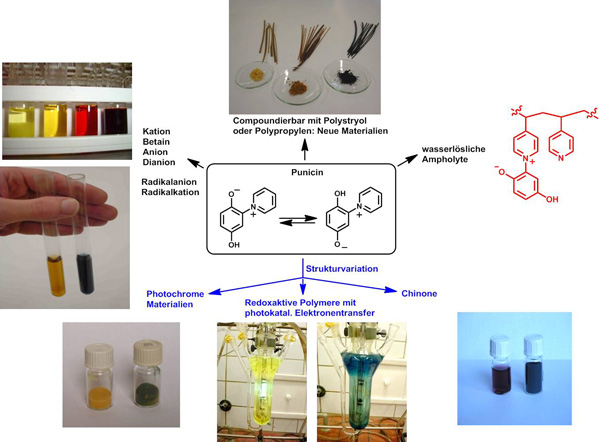
Derivatives of punicine can be used, for example, in the flotation of lithium-containing minerals, where light can be used to control the process and thus the selectivity. Our group is therefore involved in the DFG's SPP 2315, which is dedicated to the recycling of Li, Ta, Cu, and other valuable raw materials.
4. Heteroaromatic mono- to polycations as fluorescent dyes and starting materials in synthesis
Cationic, conjugated, cross-conjugated, or pseudo-cross-conjugated molecules hold great potential for surprises and are worthy of basic research. We are therefore investigating mono- to decacationic molecules that contain hetarenium substituents such as pyridinium rings, quinolinium rings, or isoquinolinium rings that are linked to each other via multiple bonds. Our investigations have led to new fluorescent dyes and extremely sensitive detection reagents, e.g., for the characterization of materials such as glass.

5. New BTE-polymer matrices for applications in nanoscopy
To overcome Abbe's law, which limits the resolution of classical light microscopes, we synthesize tailor-made bis(thienyl)ethenes (BTEs) that can be incorporated into polymer matrices for use in nanoscopy. This project is a joint project with Prof. Christian Rembe (TU Clausthal), Prof. Dr. Stefan Hell and Dr. Vladimir Belov (Max Planck Institute, Göttingen) and Prof. Alexander Egner (IFNANO Göttingen) and is funded by the DFG.
6. Heterocycle synthesis
Heterocyclic compounds are found everywhere in nature and culture, where they perform important tasks. All of the above research topics are essentially also topics from heterocyclic chemistry. Their synthesis is extremely fascinating, and we are pursuing it with great enthusiasm, for example in collaboration with Prof. Yurii Ostapiuk from Ivan Franko University in Lviv (Ukraine).
